 |
A Whirlwind Tour of Alternative Study DesignsPhilip J Clare |
1 / 36
Overview
- Introduction
- ‘Standard’ designs
- Experimental Study Designs
- Descriptive Study Designs
- Other designs
- Conclusions
2 / 36
1. Introduction
- Descriptive vs experimental
- Most important thing is to use the best design for the specific question being asked
3 / 36
1. Introduction
- Descriptive studies:
- Involve observing and assessing
- Can be used for things where it isn’t ethical to randomise
- Experimental studies:
- Involve the researchers changing something
- Tend to be more difficult and expensive
4 / 36
2. 'Standard' Designs
5 / 36
2. 'Standard' Designs
- Three standard designs we should all know:
- Randomised controlled trials
- Cross-sectional surveys
- Longitudinal surveys
- These tend to form the standard by which other study designs are considered
- eg how is a particular design better or worse than an RCT
6 / 36
2. 'Standard' Designs
Randomised Controlled Trials
- Randomise to group; give each group a different intervention; follow-up and compare the groups
- Gold standard for causation
- Ignorability
- Efficacy vs Effectiveness
- Not always appropriate
7 / 36
2. 'Standard' Designs
Randomised Controlled Trials
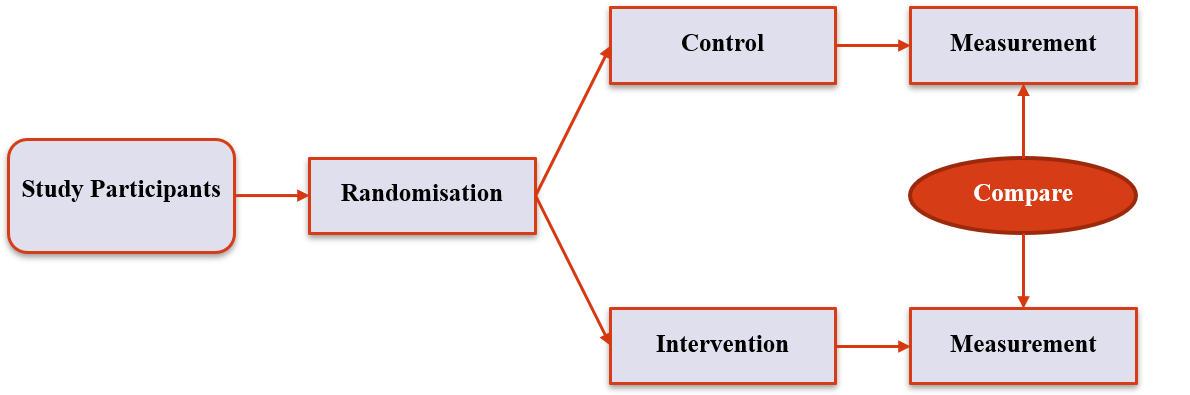
8 / 36
2. 'Standard' Designs
Cross-sectional survey
- Tends to be exploratory
- Hard to design an RCT when you don’t know what the intervention should be
- Can be cheap and easy to design, run, analyse and report
- Difficult to recruit representative samples
9 / 36
2. 'Standard' Designs
Prospective Cohort
- In some ways, just an extension of cross-sectional
- But there are a range of specific issues that arise in longitudinal research that do not apply in cross-sectional research
- There are also specific benefits
- Can be either descriptive or analytic
- With limitations, can be used for causal inference
10 / 36
2. 'Standard' Designs
Descriptive Prospective Cohort
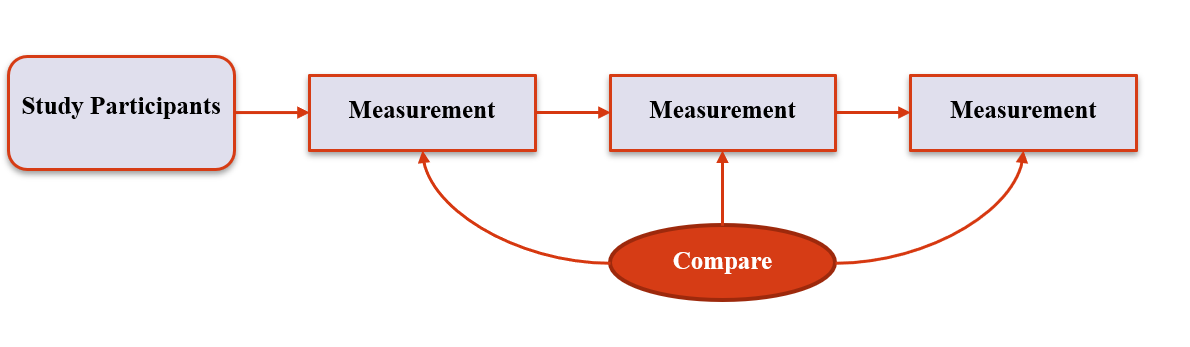
11 / 36
2. 'Standard' Designs
Analytic Prospective Cohort
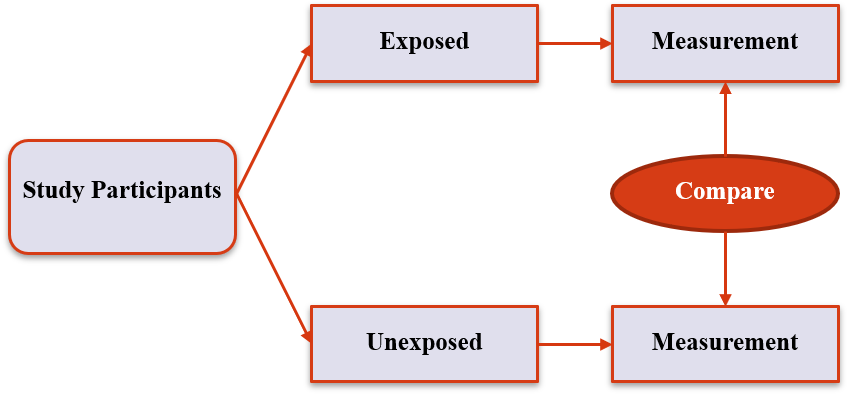
12 / 36
3. Experimental Designs
13 / 36
3. Experimental Designs
- There are lots of other experimental designs:
- Pre/post experimental trials
- Cluster RCTs
- Stepped-wedge
- Cross-over Trials
14 / 36
3. Experimental Designs
Pre/post Designs
- Measure participants, then administer intervention and measure again
- Participants form their own control
- Within subjects designs reduces variance, so requires smaller sample sizes
- Don’t know what would have happened without the experiment
15 / 36
3. Experimental Designs
Pre/post Designs
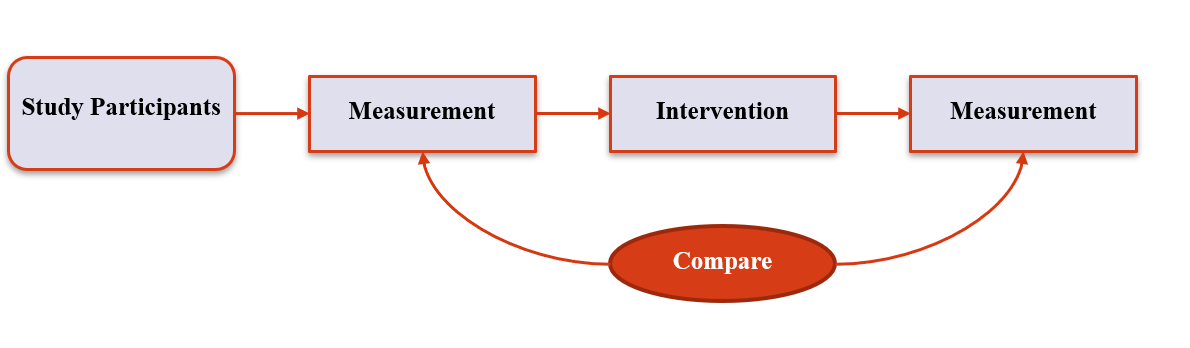
16 / 36
3. Experimental Designs
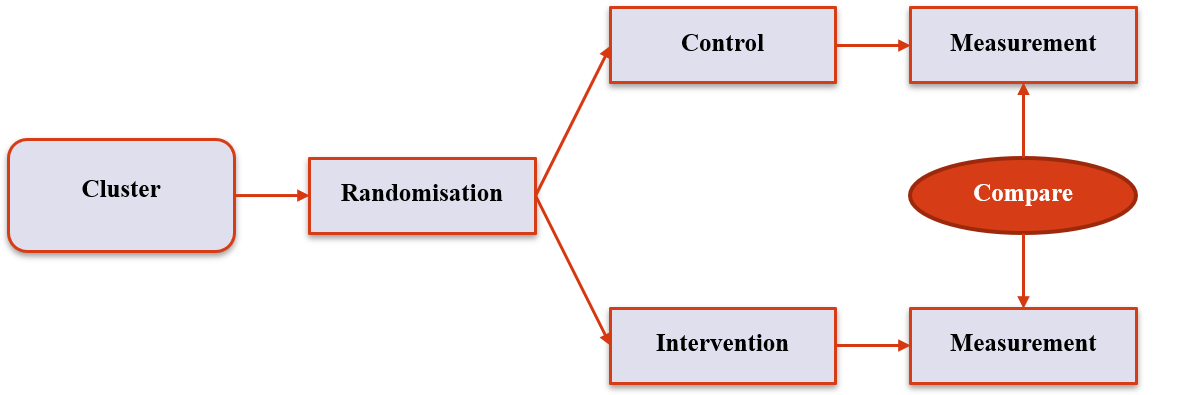
Cluster RCTs (1)
- Instead of randomisation at the individual level, randomises at the cluster eg hospital, school
- Good for systemic interventions eg new hospital process
- Also good for interventions where there is risk of cross-contamination eg participants talking to each other while in hospital
17 / 36
3. Experimental Designs
Cluster RCTs
18 / 36
3. Experimental Designs
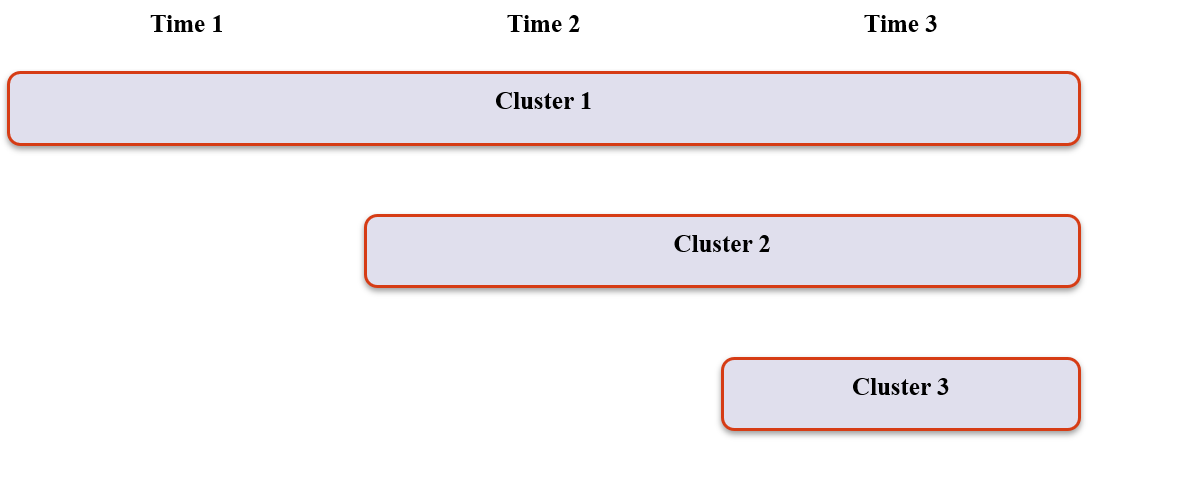
Stepped Wedge (2)
- Instead of randomising clusters to intervention or control, randomises the order that intervention begins
- Eventually rolls out to all clusters
- Pragmatic design – not great for efficacy trials
19 / 36
3. Experimental Designs
Stepped Wedge
20 / 36
3. Experimental Designs
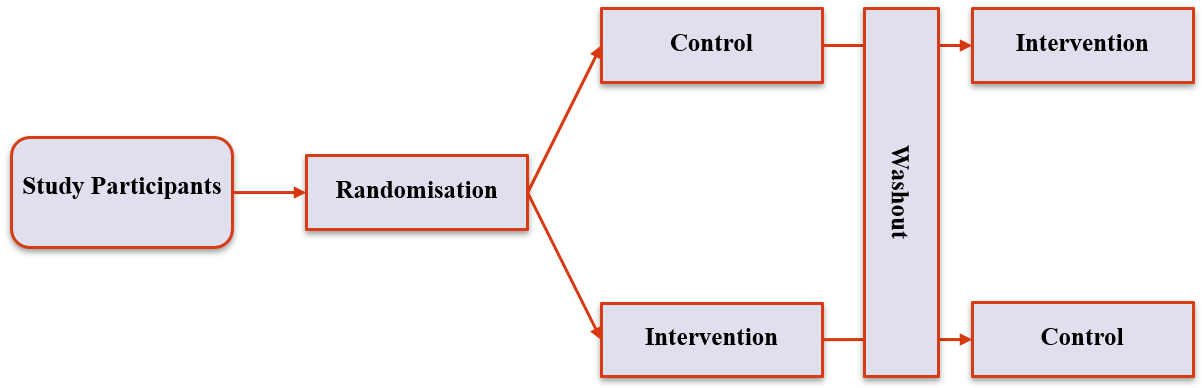
Cross-over Trials (3)
- Randomise to order; give participants intervention; wait for effect to fade; give participants intervention they didn’t receive in the first round
- Very good for experiments with multiple, short-acting interventions
- Similar benefit to pre/post – ppnts form their own control
- Allows for testing of multiple interventions
21 / 36
3. Experimental Designs
Cross-over Trials
22 / 36
4. Non-experimental Designs
23 / 36
4. Non-experimental Designs
- These include:
- Qualitative studies
- Case studies
- Case-control studies
- Ecological studies
24 / 36
4. Non-experimental Designs
Qualitative Studies
- Very in-depth
- Great for explaining WHY something happens
- Don't generalise (and aren't intended to)
25 / 36
4. Non-experimental Designs
Case Studies (4)
- In-depth
- Illustrative, rather than representative
26 / 36
4. Non-experimental Designs
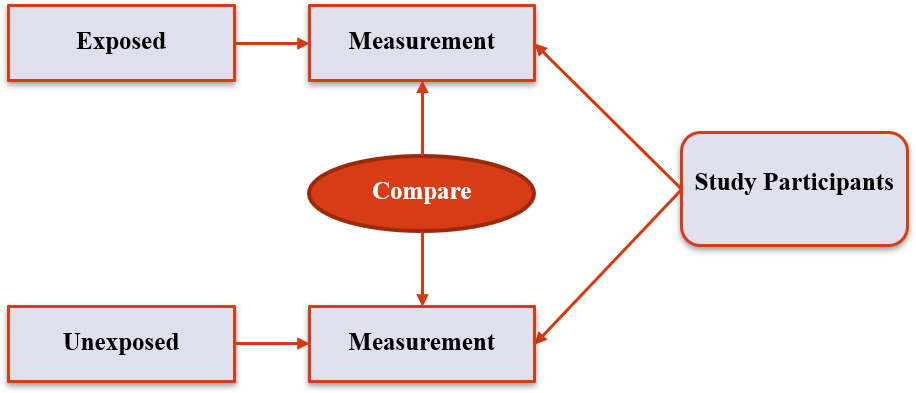
Case-control Studies (5)
- Find ‘cases’ (ie people where the outcome has occurred), then see how they differ from others in whom the outcome did not occur
- Good for rare outcomes that might not be expected to occur in a prospective study
- Can be unreliable – relies on data that has already been collected
27 / 36
4. Non-experimental Designs
Case-control Studies
28 / 36
4. Non-experimental Designs
Ecological Studies (6)
- Defined by the 'level' of measurement
- Namely, measurement is at a macro rather than individual level
- Can be used to measure very rare diseases etc
- Can lead to the 'ecological fallacy'
- Where the nature of the group is falsely used to describe the nature of individuals in the group
29 / 36
5. Other Designs
30 / 36
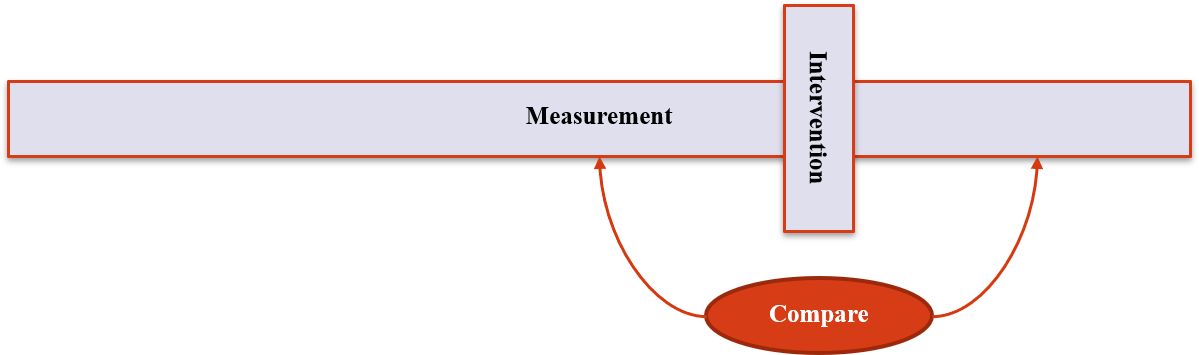
5. Other Designs
Interrupted Time Series (7)
- Measures the change in a trend that is ‘interrupted’
- Technically involves an intervention – but often out of the researchers hands
- Sometimes the only way to assess whether high-level interventions have any effect.
- Can be problematic because effects often aren't instant
31 / 36
5. Other Designs
Interrupted Time Series
32 / 36
5. Other Designs
Linkage Studies (8)
- Can be either descriptive or experimental
- Can involve linking administrative data to an existing study such as a cohort study, or linking different administrative datasets to each other
- Can be very complex, and involve extremely large datasets
33 / 36
6. Conclusions
- There are lots of study designs that can be used
- Most important thing is to use the best design for the specific question being asked
34 / 36
7. References
- Murray DM, Varnell SP, Blitstein JL. Design and Analysis of Group-Randomized Trials: A Review of Recent Methodological Developments. Am J Public Health. 2004;94(3):423-432.
- Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350:h391.
- Johnson DE. Crossover experiments. WIREs Comp Stat. 2010;2:620-625.
- Yin RK. Case study research: design and methods. SAGE Publications. 2013.
- Mann CJ. Observational research methods. Research design II:cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20:54-60.
35 / 36
7. References
- Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annu Rev Public Health. 1995;16:61-81.
- Kontopantelis E. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350:h2750.
- Bohensky MA, Jolley D, Sundararajan V, Evans S, Pilcher DV, Scott I, Brand CA. Data Linkage: A powerful research tool with potential problems. BMC Health Services Research. 2010;10:346.
36 / 36